# Major Recall Alert: ShockPulse Lithotripsy Generator, ShockPulse-SE Lithotripsy System Recalled Due to Potential Mis-Wired Components
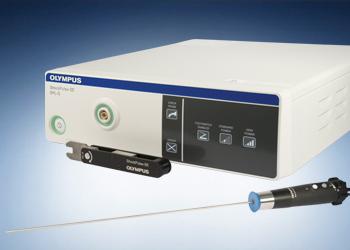
Olympus has announced a product recall affecting specific serial numbers of their **ShockPulse Lithotripsy Generator** and **ShockPulse-SE Lithotripsy System**. This action comes after an internal investigation revealed a potential issue with mis-wired components in certain devices. Users are urged to check their equipment and contact Olympus customer service to arrange for repairs. Below, we share all the critical information you need to know about this recall and how to stay safe.
---
## Why This Recall is Important
The **ShockPulse Lithotripsy Generator** and **ShockPulse-SE Lithotripsy System** are high-precision medical devices used in lithotripsy, a procedure to break down kidney stones. These devices must operate seamlessly to ensure the safety of both patients and healthcare professionals.
Olympus has identified that some units of these systems may have a **mis-wired component**—a defect that could compromise the device's performance or even pose a safety risk. Though no incidents or injuries have been reported so far, addressing this issue promptly is critical to maintaining medical safety standards.
Healthcare facilities and device users are strongly advised to follow the recall instructions to minimize potential risks.
---
## Details of the Recall
Here’s what you need to know about the recall:
- **Brand**: Olympus
- **Affected Products**:
- *ShockPulse Lithotripsy Generator*
- *ShockPulse-SE Lithotripsy System*
- **Reason for Recall**: Internal investigations discovered potential **mis-wired components** in specific serial numbers of the devices.
- **Announced Date**: [Insert Official Date from Recall Source]
- **Safety Risk**: Faulty wiring could impact device functionality, which may lead to delayed or suboptimal medical care.
You can view the official recall notice issued by Health Canada here: [Official Recall Notice](https://recalls-rappels.canada.ca/en/alert-recall/shockpulse-se-lithotripsy-system-and-shockpulse-lithotripsy-generator).
---
## What You Should Do
If you own or use the ShockPulse Lithotripsy Generator or ShockPulse-SE Lithotripsy System, follow these steps immediately:
- **Step 1**: Check the serial number of your device to determine if it is part of the recall.
- **Step 2**: Contact **Olympus customer service** to report your device and arrange for its return and repair. Olympus will provide further instructions on how to proceed.
- **Step 3**: Discontinue use of the device until it is inspected and repaired by Olympus.
Ensuring prompt action will help safeguard the safety of patients and users alike.
---
## Stay Safe – Get Instant Recall Alerts
Staying informed about product recalls, especially for high-stakes devices like medical equipment, is critical. Install the **MyRecalls App** today to receive real-time alerts on recalls affecting your products. With instant notifications and easy access to recall details, you can stay one step ahead in ensuring safety for yourself and others.
👉 **[Download the MyRecalls App Now](#)**
For additional details about this recall, check out the official notice from Health Canada here: [Official Health Canada Recall Notice](https://recalls-rappels.canada.ca/en/alert-recall/shockpulse-se-lithotripsy-system-and-shockpulse-lithotripsy-generator).
Spread the word to ensure no one is left unaware of this critical recall. Your proactive response matters!
Stay informed, stay safe, and act swiftly!