# Major Recall Alert: MAMMOMAT Inspiration Recalled Due to Operator Table Safety Risk
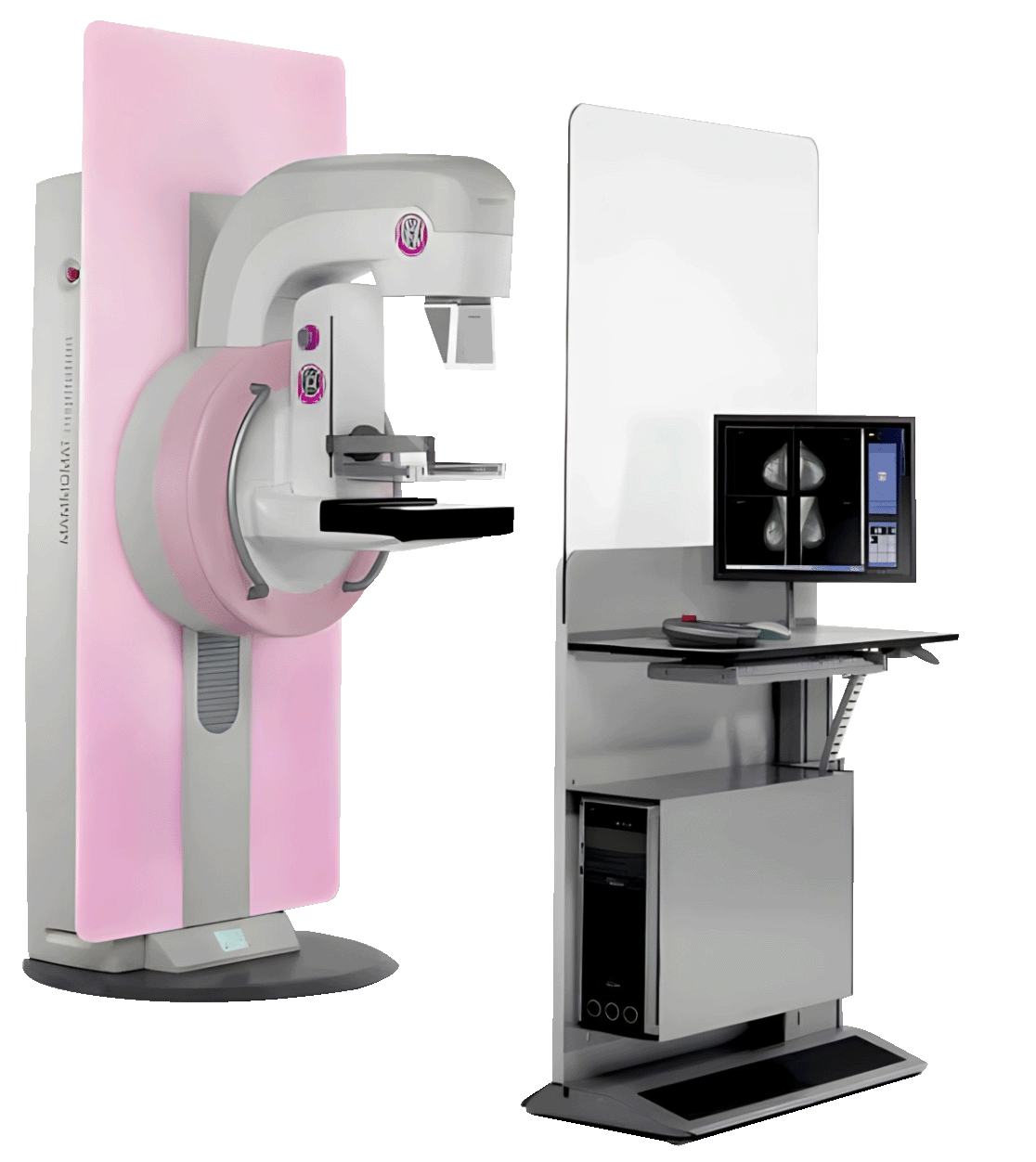
The MAMMOMAT Inspiration, a medical imaging product, has been officially recalled due to risks associated with its operator table (part number 14467741). This table, originally designed for stationary use, was not intended or approved for bus installations. During transportation, repeated vibrations and impacts could cause components such as screws, the monitor, or its holder to loosen, posing a serious safety risk to operating staff. Additionally, potential damage to the lead shield further increases risk, making this issue critical to address. Learn more about this recall and what steps you need to take below.
## Why This Recall is Important
This recall has highlighted a serious design mismatch involving the operator table of the MAMMOMAT Inspiration, designed solely for stationary use but inadvertently installed in buses.
- **Potential Safety Hazards**: Vibrations during transportation could result in components, including screws, the monitor, or its holder, loosening and potentially falling. This creates a severe risk of injury to operators.
- **Additional Concerns**: Damage to the lead shield, critical for safe operation, may also occur, raising potential health hazards.
Safety is paramount, particularly in medical environments, where reliable equipment is essential for both operator and patient safety. This recall serves as an important reminder to manufacturers and healthcare providers to ensure proper use and installation of medical devices.
Consumers must act promptly if their MAMMOMAT Inspiration operator table is involved in this recall to mitigate potential risks.
## Details of the Recall
The recall targets the operator table of the **MAMMOMAT Inspiration**. Here’s what you need to know:
- **Brand**: MAMMOMAT Inspiration
- **Issue**: Operator table (part number 14467741) is unsafe for bus installations due to its design for stationary operation only.
- **Risks**: Vibrations and impacts during transportation could cause components to loosen and fall, resulting in a risk of serious injury. Additionally, the lead shield may sustain damage.
- **Date Announced**: The recall was issued on [insert date here].
- **Image**:
You can find the official recall notice published by the **Government of Canada** [here](https://recalls-rappels.canada.ca/en/alert-recall/mammomat-inspiration).
## What You Should Do
If you own, operate, or rely on a MAMMOMAT Inspiration operator table, it’s important to take swift action to protect your safety and that of others:
- **Stop Using the Device Immediately**: If the operator table is installed in a bus or mobile environment, cease use immediately.
- **Inspect the Device**: Check for signs of loose screws, monitor instability, or potential lead shield damage.
- **Contact the Manufacturer**: Reach out to the manufacturer for guidance or to arrange for repairs, replacements, or refunds as applicable.
- **Follow Recall Instructions**: Adhere to the official steps outlined in the [recall announcement](https://recalls-rappels.canada.ca/en/alert-recall/mammomat-inspiration).
If you’re unsure whether your device is affected, consult the product label and packaging or contact MAMMOMAT customer support.
## Stay Safe – Get Instant Recall Alerts
Product recalls like this emphasize the importance of staying informed about potential risks associated with consumer and medical products. To make this easier, download the **MyRecalls App** today.
By using **MyRecalls**, you’ll:
- Receive **instant alerts** about critical recalls.
- Access detailed recall information and guidance.
- Keep your family, employees, and patients safe with timely updates.
[**Download the MyRecalls App Now**](#) to ensure you’re always in the know about vital product safety updates.
Your safety matters. Act now to prevent injury and ensure compliance with recall instructions.
Stay informed. Stay safe.