# Major Recall Alert: LOGIQ P10 Ultrasound Systems Recalled Due to Inaccurate Liver Steatosis Data on Version R4.5.7 Software
GE Healthcare has issued an important recall for certain LOGIQ P9 and LOGIQ P10 ultrasound systems that operate on system software version R4.5.7. The issue concerns inaccurate data from the Ultrasound-Guided Attenuation Parameter (UGAP) measurement, which plays a critical role in diagnosing liver steatosis. If you're a user of the affected systems, here's what you need to know to stay informed and ensure your safety.
## Why This Recall is Important
The LOGIQ P10 ultrasound system is widely used in medical imaging, particularly for diagnosing liver conditions such as fatty liver (liver steatosis). Accurate UGAP readings are crucial for evaluating liver health, guiding clinical decisions, and determining treatment plans. GE Healthcare has discovered that with software version R4.5.7, UGAP measurement data may present **inaccurate values**. This poses serious risks, including potential misdiagnosis or improper medical care for liver conditions.
Failure to act on this recall could lead to delayed or incorrect treatments, putting patients’ health at significant risk. GE Healthcare has made this recall public to prevent harm and ensure that healthcare providers using these devices are informed.
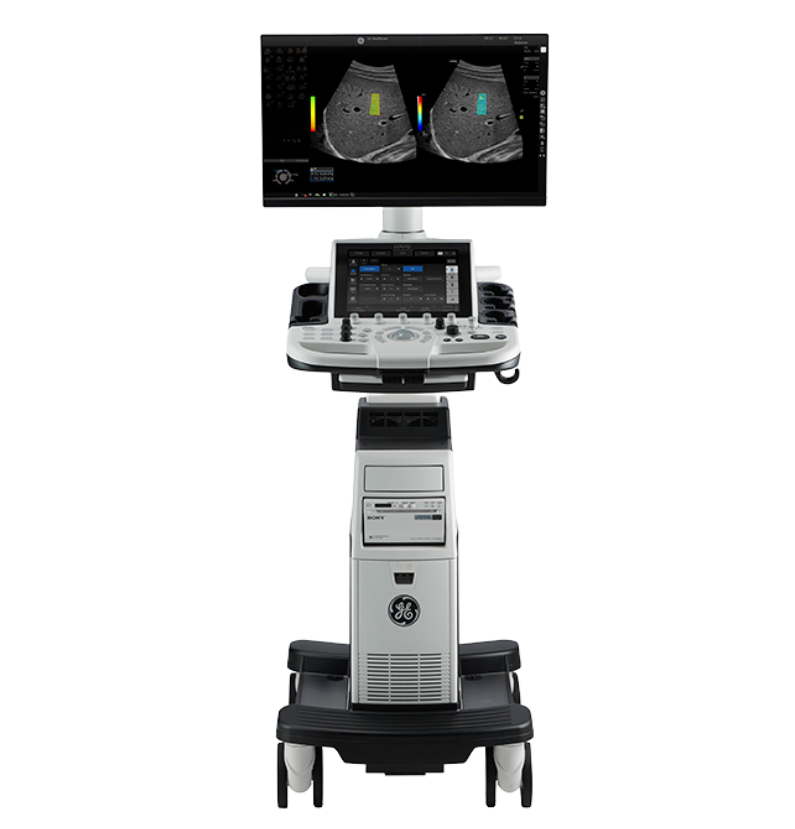
## Details of the Recall
Here are the key details regarding the LOGIQ P10 recall:
- **Category**: Medical ultrasound systems
- **Affected Brand and Product**: LOGIQ P9 and LOGIQ P10
- **Software Issue**: System software version **R4.5.7**
- **Reason for Recall**: Ultrasound-Guided Attenuation Parameter (UGAP) data may display inaccurate results for liver steatosis.
- **Date Announced**: 30205
- **Source**: [Official Recall Notice from GE Healthcare](https://recalls-rappels.canada.ca/en/alert-recall/logiq-p10)
It's essential for healthcare providers, clinics, and hospitals using LOGIQ P9 or LOGIQ P10 systems to identify if their devices are operating on the affected software version.
## What You Should Do
If you own or operate a LOGIQ P10 or LOGIQ P9 ultrasound system with software version R4.5.7, here are the steps recommended by GE Healthcare:
- **Stop Using the UGAP Function**: Avoid relying on UGAP measurements for liver steatosis diagnosis until the issue is resolved.
- **Contact GE Healthcare**: Reach out to the support team for instructions on updating or fixing the system.
- **Check Your Device Software**: Confirm your ultrasound system's software version to verify whether it's affected by this recall.
GE Healthcare is actively addressing this issue and will provide guidance on how to resolve the software error.
## Stay Safe – Get Instant Recall Alerts
Medical recalls can happen unexpectedly, directly impacting patient safety and care quality. Don’t let critical updates slip through the cracks—stay ahead with fast and reliable recall notifications.
Download the [MyRecalls App](https://recalls-rappels.canada.ca/en/alert-recall/logiq-p10) to receive **real-time alerts** on product recalls. With the app, you can easily track recalls, confirm if your equipment or devices are impacted, and access manufacturer instructions for corrective measures.
Stay informed, take action, and prioritize safety with MyRecalls. Click the link to [download the app today](https://recalls-rappels.canada.ca/en/alert-recall/logiq-p10)!
### Final Thoughts
The LOGIQ P10 and LOGIQ P9 ultrasound systems recall highlights the importance of maintaining up-to-date software and prompt responses to manufacturer alerts. If you or your facility is affected, take the necessary steps outlined above to ensure patient safety and the integrity of your diagnostics. For more information, check the [official recall notice here](https://recalls-rappels.canada.ca/en/alert-recall/logiq-p10).