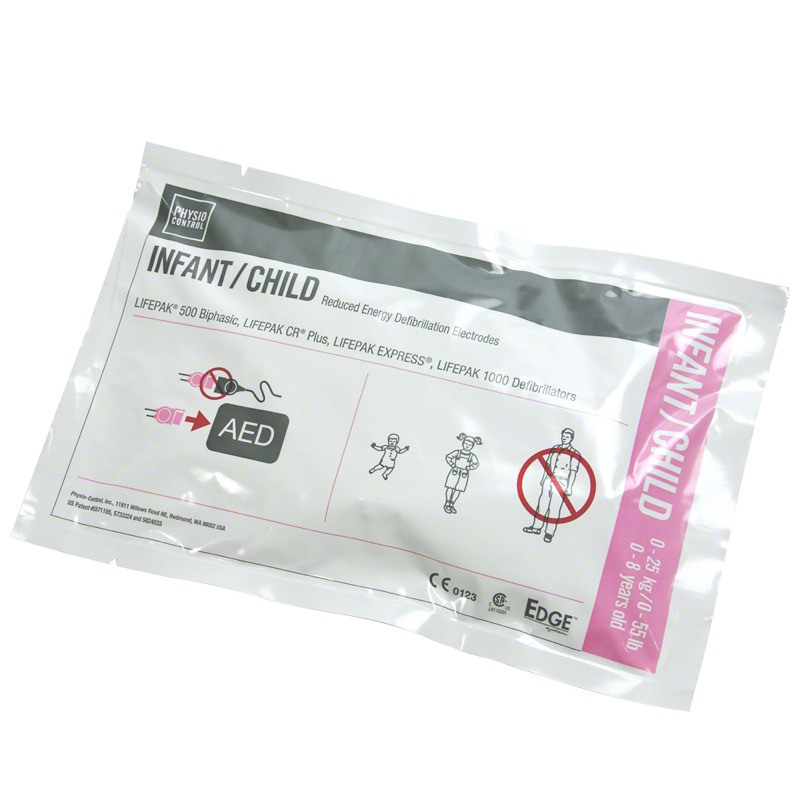
# Major Recall Alert: Infant/Child Reduced Energy Defibrillation Electrode Recalled Due to Gel Delamination Concerns
Stryker, a renowned medical device manufacturer, has issued a critical recall of its Infant/Child Reduced Energy Defibrillation Electrode. This urgent recall comes in response to reported complaints about gel delamination, which could compromise the performance of these life-saving pediatric electrodes. Here’s everything you need to know about this recall, why it matters, and how you can stay informed.
## Why This Recall is Important
Defibrillation electrodes play a vital role in helping healthcare providers and emergency responders deliver electric shocks to restore normal heart rhythms, especially in infants and children. Any defect in these devices could pose a significant risk to patient safety.
The primary concern in this recall centers around **gel delamination**, a manufacturing issue that can impair the adhesive qualities of the electrode. This could result in improper device function or difficulty delivering a precise, effective shock during critical medical situations.
The issue, first investigated by Stryker on **November 21, 2024**, has raised red flags in hospitals, clinics, and emergency responders across Canada. It is absolutely essential for caregivers, healthcare providers, and parents to take this recall seriously to prevent potential complications in emergency treatment scenarios.
## Details of the Recall
Here’s a quick summary of the recall details:
- **Category**: Medical Device Recall
- **Manufacturer**: Stryker
- **Product**: Infant/Child Reduced Energy Defibrillation Electrode
- **Date of Investigation**: November 21, 2024
- **Reason for Recall**: Complaints regarding gel delamination issues compromising product effectiveness
- **Affected Consumers**: Healthcare providers, hospitals, clinics, and emergency response teams using this product for pediatric patients
For more official information, you can visit the Government of Canada’s recall website [here](https://recalls-rappels.canada.ca/en/alert-recall/infantchild-reduced-energy-defibrillation-electrode).
## What You Should Do
If you or your organization uses the **Infant/Child Reduced Energy Defibrillation Electrode**, follow these safety measures immediately:
- **Discontinue Use**: Stop using the affected product immediately.
- **Check Inventory**: Inspect medical storage areas to identify any Infant/Child Reduced Energy Defibrillation Electrodes with potential issues.
- **Contact Stryker**: Reach out to the manufacturer to report the defective product and obtain guidance on replacements or refunds.
- **Alert Your Team**: Inform all relevant medical staff about the recall to avoid inadvertent use of the affected electrodes.
For additional assistance, consult the **Stryker product support team** or your supplier. It’s crucial to ensure no affected electrodes remain in circulation.
## Stay Safe – Get Instant Recall Alerts
Recalls like this highlight how critical it is to stay informed about product safety, especially when it comes to medical devices used in emergencies. Don’t wait until it’s too late—stay one step ahead!
Download the [MyRecalls app](https://myrecalls.app) today to receive real-time alerts about recalls impacting your household and workplace. With the app, you can:
- Track recalls for medical devices, food, electronics, and more
- Get notifications instantly when safety issues are reported
- Access detailed information and safety instructions for each recall
Stay informed, stay safe, and protect your loved ones.
---
**Act now** to ensure compliance with safety standards and help prevent potential harm. Visit the [official recall page](https://recalls-rappels.canada.ca/en/alert-recall/infantchild-reduced-energy-defibrillation-electrode) for complete details, or download the app for continued updates on product recalls.