# Major Recall Alert: Healmoxy Capsules 500mg Recalled Due to Falsified Batches
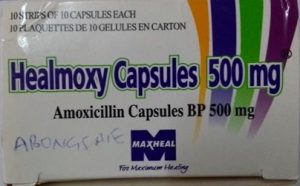
In a significant public health update, the National Agency for Food and Drug Administration and Control (NAFDAC) has issued a recall for falsified batches of **Healmoxy Capsules 500mg**, manufactured by **Maxheal Pharmaceuticals**. These fake medicines lack the **active pharmaceutical ingredient (API)** needed for efficacy and feature **inconsistent manufacturing and expiry dates**, posing potential risks to users.
Consumers, healthcare providers, and supply chain stakeholders must remain vigilant. Learn more about this recall, its implications, and how to stay safe below.
---
## Why This Recall is Important
The recall of Healmoxy Capsules 500mg highlights critical dangers within the pharmaceutical supply chain:
1. **Absence of API**: These falsified medicines lack the active pharmaceutical ingredient required to effectively treat health conditions, making them ineffective and dangerous for patients relying on them for recovery.
2. **Tampered Labeling**: The inconsistent manufacturing and expiry dates on the falsified batches make it impossible for consumers to verify the product’s authenticity or shelf life.
3. **Public Health Risk**: The widespread circulation of substandard drugs increases the risk of treatment failure, adverse effects, and long-term damage to patients' health.
According to NAFDAC, the falsified Healmoxy Capsules have been detected in **Cameroon** and the **Central African Republic (CAR)**, but the distribution could extend further. Anyone who may have purchased or received these capsules should take immediate action to ensure their safety.
Read the full announcement here: [NAFDAC Official Recall Notice](https://nafdac.gov.ng/public-alert-no-17-2025-alert-on-falsified-batches-of-healmoxy-capsules-500mg-found-in-cameroon-and-central-african-republic-car/).
---
## Details of the Recall
### Product Affected:
- **Brand**: Maxheal Pharmaceuticals
- **Product**: Healmoxy Capsules 500mg
- **Reason for Recall**:
- Lack of active pharmaceutical ingredient (API)
- Inconsistent manufacturing and expiry date formats
### Announced By:
- **Agency**: National Agency for Food and Drug Administration and Control (NAFDAC)
- **Countries Impacted**: Cameroon and Central African Republic (CAR)
### Safety Concerns:
- The tampered medicines may lead to treatment failure or exacerbate existing health conditions.
- Patients taking this product are at heightened risk of consuming a completely ineffective drug.
---
## What You Should Do
If you suspect you have falsified Healmoxy Capsules 500mg, **take these actions immediately**:
- **Check the Product**: Verify manufacturing and expiry dates listed on the package. Be cautious of inconsistent or unclear labeling.
- **Report to Authorities**: Notify the nearest **NAFDAC office** for further guidance. Reports can also be sent through their contact details provided on [NAFDAC's official website](https://nafdac.gov.ng).
- **Source Medications Properly**: Only purchase medicines from **authorized or licensed suppliers** to avoid counterfeit products.
- **Inspect Products Carefully**: Always check a medicine’s physical condition, including seal integrity, labeling, and expiration date before use.
---
## Stay Safe – Get Instant Recall Alerts
Counterfeit medications pose a serious threat to your health and safety. To stay informed on critical recall alerts like this, download NAFDAC's mobile app for real-time updates, tips for identifying substandard products, and direct reporting channels.
👉 **[Download the NAFDAC App Here](https://nafdac.gov.ng)**
Your health and safety are important—stay vigilant and informed with NAFDAC as your trusted ally.
---
Keep yourself and your loved ones protected by remaining cautious about the medicines you consume. For additional details and official updates, visit NAFDAC's recall notice here: [Official Recall Notice](https://nafdac.gov.ng/public-alert-no-17-2025-alert-on-falsified-batches-of-healmoxy-capsules-500mg-found-in-cameroon-and-central-african-republic-car/).