# Major Recall Alert: Flexor® Check-Flo Introducer Sets and Rösch-Uchida Transjugular Liver Asset Set Recalled Due to Manufacturing Issues
Cook Medical has issued an urgent recall for multiple medical device products, including the **Flexor® Check-Flo Introducer Sets** and **Rösch-Uchida Transjugular Liver Asset Set**. The recall is the result of products being manufactured out of specification, potentially leading to life-threatening complications during use. Impacted devices may have been cut to incorrect lengths, not properly trimmed, and/or inadequately inspected before distribution.
If you or someone you know uses these devices, it’s essential to understand the risks, details of the recall, and necessary next steps to ensure patient safety.
## Why This Recall is Important
Medical devices are crucial instruments in surgical and diagnostic procedures, meaning even minor defects can lead to severe repercussions. **Cook Medical’s Flexor® Check-Flo introducers and Rösch-Uchida sets** are widely used in various critical medical applications, such as vascular access, liver procedures, and more. The issues identified include:
- **Device length deficiencies** – Incorrectly cut devices may affect their functionality.
- **Improper trimming** – Edges not trimmed properly could cause injury during use.
- **Lack of adequate inspection** – Unchecked defective devices pose significant procedural risks.
These defects raise serious concerns, as malfunctioning devices might compromise patient outcomes. Regulatory bodies take such recalls seriously to safeguard public health.
## Details of the Recall
Here’s everything you need to know:
- **Products Affected:**
- Flexor® Check-Flo II Introducer Set
- Flexor® Check-Flo Introducer Set
- Flexor® Check-Flo Introducer-Ansel 1 Modification-With High-Flex Dilator and Hydrophilic Coating
- Flexor® Check-Flo Introducer-Ansel Modification
- Flexor® Check-Flo Introducer-Ansel Modification-With Highflex Dilator and Hydrophilic Coating
- Flexor® Check-Flo Introducer-Raabe Modification
- Rösch-Uchida Transjugular Liver Access Set
- **Recall Reason:**
Cook Medical identified that these products may have been made out of specification, potentially leading to the following issues:
- Incorrect lengths
- Untidy trimming
- Lack of thorough inspection
- **Date Announced:** October 2023
- **Image of Affected Products:**
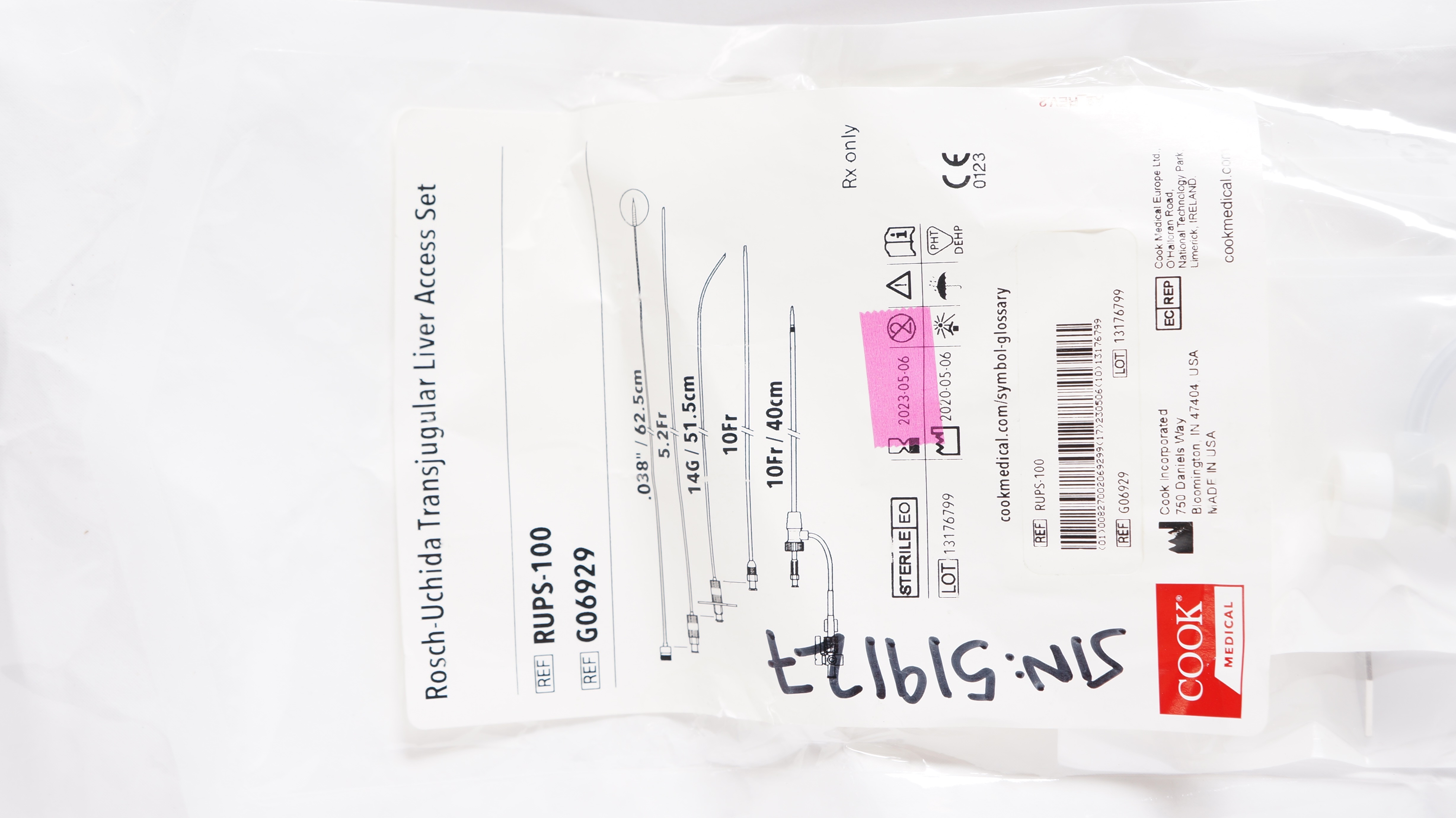
Consumers and medical professionals should discontinue the use of these products immediately and follow Cook Medical’s outlined steps for replacement or refund.
For more detailed information, visit the official recall announcement [here](https://recalls-rappels.canada.ca/en/alert-recall/flexorr-check-flo-introducer-and-rosch-uchida-transjugular-liver-access-sets).
## What You Should Do
If you’re a medical professional or healthcare facility:
1. **Check Your Inventory:** Confirm whether you have any of the affected devices in stock.
2. **Discontinue Use Immediately:** Do not use any products that are part of the recalled lot.
3. **Contact Cook Medical:** Reach out to Cook Medical to arrange for replacements, refunds, or further instructions.
4. **Communicate with Patients:** If these devices were used in any recent procedures, closely monitor patient outcomes and consult with experts if necessary.
For individuals:
- Consult your healthcare provider if you believe an affected device may have been used during a procedure.
- Remain vigilant about any unusual symptoms and inform your doctor immediately.
## Stay Safe – Get Instant Recall Alerts
Product recalls can happen unexpectedly. To stay safe and informed, download the [MyRecalls app](https://myrecalls.app) today. With real-time notifications and updates, you’ll never miss critical product safety announcements that could affect your health or safety.
Cook Medical’s recall highlights the importance of always staying informed about the tools and devices involved in your healthcare. Don’t wait—act now to protect yourself and others.
_Tagged with keywords: Flexor Check-Flo recall, Cook Medical manufacturing defects, medical device recall October 2023, Rösch-Uchida set recall, medical safety recall, product recall Canada._