# Major Recall Alert: DuraSeal Dural Sealant Recalled Due to Missing Age Restriction on Canadian IFU
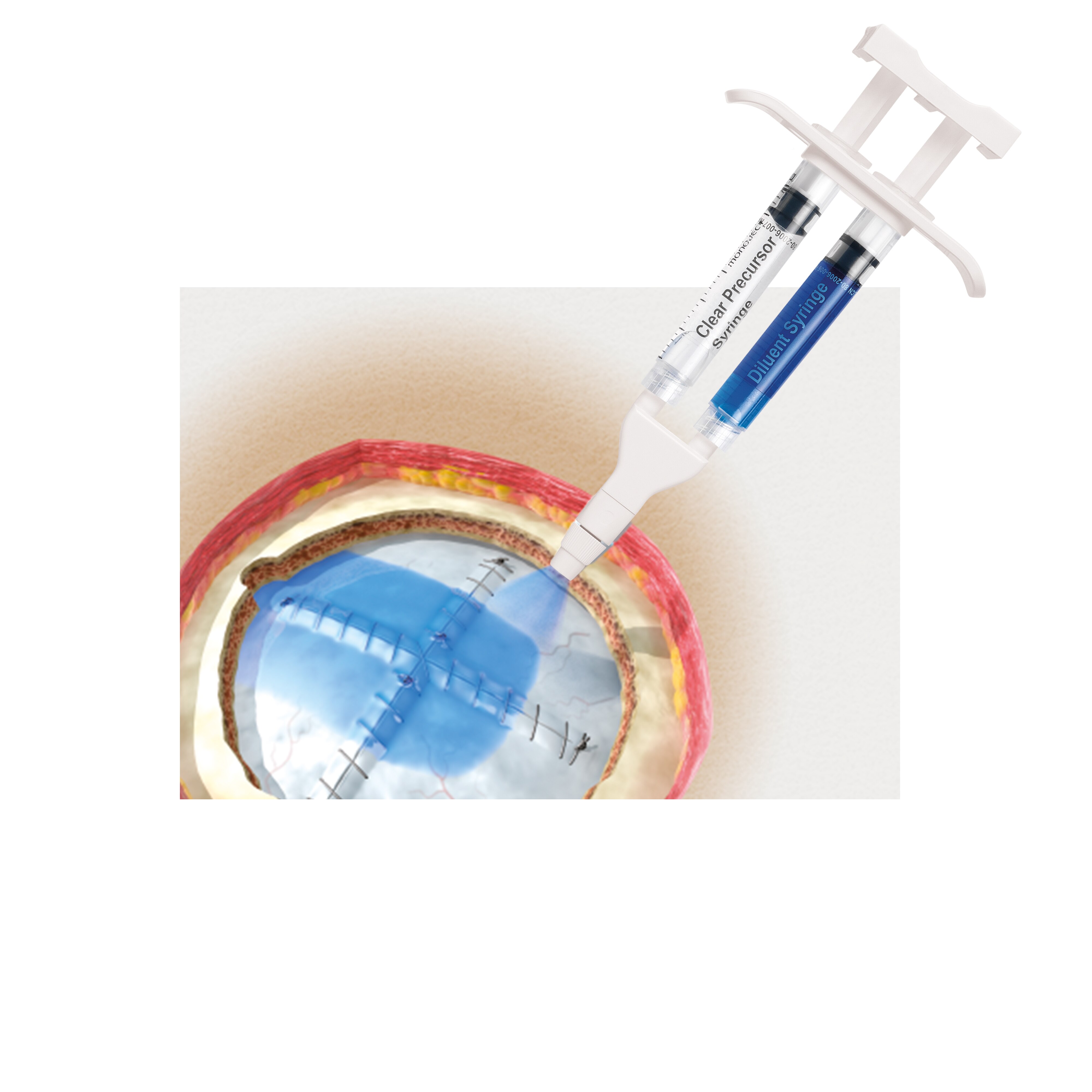
Consumers and healthcare professionals across Canada should take note. On **March 13, 2025**, a recall was issued for **DuraSeal Dural Sealant**, a medical product widely used in surgical procedures involving the central nervous system. The recall stems from a critical oversight in the Canadian Instructions for Use (IFU). Specifically, an age restriction was missing, instructing that this product should not be used in patients under **13 years of age**.
## Why This Recall is Important
The **DuraSeal Dural Sealant** is commonly used for sealing dural tears after surgery. While generally safe and effective when used for appropriate patients, a thorough investigation revealed a significant gap in the product's Canadian guidelines.
An **age restriction for patients younger than 13 years old** was absent from the IFU, which could lead to potential use in younger patients. Such an oversight may expose children under 13 to unforeseen health risks due to their unique physiological conditions and compatibility concerns with the product.
The inclusion of precise usage instructions and precautions on medical products is crucial to guarantee patient safety and informed decision-making by healthcare providers. This recall ensures that the necessary directive—"Precaution: do not use in patients younger than 13 years of age"—is added to all Canadian IFUs.
## Details of the Recall
Key information regarding the **DuraSeal Dural Sealant recall**:
- **Brand**: DuraSeal Dural Sealant
- **Product**: DuraSeal Dural Sealant System
- **Recall Start Date**: March 13, 2025
- **Reason for Recall**: Missing age restriction ("do not use in patients younger than 13 years of age") from the Canadian Instructions for Use (IFU).
- **Issue Identified Through**: Internal investigation and review of supporting evidence.
- **Potential Impact**: Use in younger patients may result in unintended health risks.
For full details, visit the official recall announcement [here](https://recalls-rappels.canada.ca/en/alert-recall/duraseal-dural-sealant).
## What You Should Do
If you or your healthcare facility uses **DuraSeal Dural Sealant**, it is critical to take immediate action:
- **Cease use in patients under the age of 13**: Ensure compliance with the updated age restriction for the product.
- **Review existing stock**: Verify whether the product includes the revised Canadian IFU with the age restriction.
- **Notify appropriate personnel**: Alert all relevant medical professionals and staff about the recall details to prevent unauthorized use.
- **Contact the manufacturer**: For any concerns, or to request updated IFUs, reach out to the product manufacturer.
If this product was used in a patient under 13 and adverse symptoms arise, consult a medical professional immediately and report the incident to Health Canada’s **Recalls and Safety Alerts** division.
## Stay Safe – Get Instant Recall Alerts
With medical product recalls increasing, it’s essential to stay informed about products affecting your health and well-being. Download the [MyRecalls App](https://myrecalls.app) to:
- Receive **instant notifications** on product recalls in Canada and globally.
- Access detailed information to protect your family and patients.
- Stay up to date on the latest safety advisories.
Don’t leave your safety to chance—be proactive and informed.
## Final Thoughts
The recall of **DuraSeal Dural Sealant** highlights the importance of accurate product labeling, especially for medical devices. If you or someone you know uses this product, ensure immediate precautions are taken to adhere to the updated age restriction guidelines. For more guidance, refer to [Health Canada’s official recall notice](https://recalls-rappels.canada.ca/en/alert-recall/duraseal-dural-sealant).
Stay vigilant. Stay safe. Get recall alerts today.