# Major Recall Alert: Broselow™/Hinkle Pediatric Emergency System Emergency Tape, Broselow™/Hinkle Pediatric Emergency System Kit Recalled Due to Medication Dosage Errors
AirLife™ has issued a recall of the Broselow™/Hinkle Pediatric Emergency System Emergency Tape and Kit after discovering critical medication dosage errors printed on its Rainbow Tape. These errors, specifically in the dosages for **ketamine, flumazenil, and vecuronium**, pose significant safety risks and could lead to adverse events during pediatric emergency care. This recall affects healthcare providers and facilities relying on these products for accurate dosing during critical situations.
Read on to learn the details of this urgent recall and what you can do to stay safe and informed.
---
## Why This Recall is Important
Medical emergencies require absolute accuracy, especially when treating pediatric patients. The **AirLife™ Broselow™ Rainbow Tape** is a key tool in the Broselow™/Hinkle Pediatric Emergency System, designed to facilitate quick and accurate drug dosages based on children’s estimated weight and height.
However, AirLife™ recently found alarming medication dosage discrepancies in the affected products, including errors in:
- **Ketamine dosage** (commonly used for sedation)
- **Flumazenil dosage** (a reversal agent for benzodiazepines)
- **Vecuronium dosage** (a neuromuscular blocking agent)
Mistakes related to these medicines could cause severe harm, including ineffective treatment, adverse reactions, or life-threatening outcomes in pediatric emergency settings. This highlights the critical need for swift action to remove the affected versions and prevent any potential adverse events.
---
## Details of the Recall
Here’s what you need to know about the **AirLife™ Broselow™ Rainbow Tape recall**:
- **Affected Brand**: Broselow™/Hinkle Pediatric Emergency System
- **Product Names**:
- Broselow™/Hinkle Pediatric Emergency System Emergency Tape
- Broselow™/Hinkle Pediatric Emergency System Kit
- **Issue Identified**: Errors in medication dosages listed on the tape (ketamine, flumazenil, vecuronium)
- **Potential Risks**: Incorrect medication administration could lead to adverse medical events or harm in emergency situations.
- **Date Announced**: October 9, 2023
- **Image of Product**:
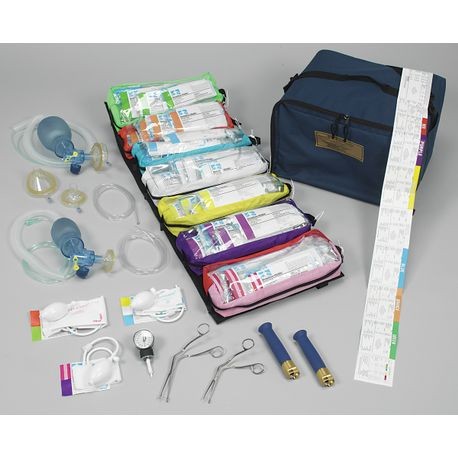
For more information, refer to the **official source** [here](https://recalls-rappels.canada.ca/en/alert-recall/broselowtmhinkle-pediatric-emergency-system-sets).
---
## What You Should Do
If your facility uses the affected Broselow™/Hinkle products, it’s critical to take the following steps immediately:
1. **Stop Using the Recalled Products**: Cease using the affected Rainbow Tape and Kits in all emergency scenarios.
2. **Separate and Dispose of Faulty Items**: Ensure the recalled products are segregated and not accessible for use.
3. **Replace with Updated Products**: Contact AirLife™ or your distributor for guidance on obtaining updated versions with corrected dosages.
4. **Report Adverse Events**: If an incident related to this recall has occurred, report it to Health Canada or your local health authority.
Taking prompt action ensures patient safety and avoids potential errors in critical situations.
---
## Stay Safe – Get Instant Recall Alerts
Medical recalls like this highlight the importance of staying informed about the latest product safety notices. To ensure you never miss critical updates:
1. **Download the MyRecalls App**: Our app gives real-time alerts about recalls for medical tools, drug information, and other safety notices.
2. **Track and Manage Recalls**: Easily track products you use and stay updated with solutions provided by manufacturers.
3. **Protect Patients, Customers, and Loved Ones**: Stay proactive about safety and ensure swift action on all recall notifications.
**[Download the MyRecalls App Now](https://myrecalls.app/download)** and be the first to know about essential recalls that matter to you.
---
Keeping our communities safe in emergencies starts with accurate, reliable tools. Stay vigilant, stay informed, and act immediately by removing affected AirLife™ Broselow™/Hinkle products from circulation.
For the official recall notice or more details, visit the Health Canada website:
[https://recalls-rappels.canada.ca/en/alert-recall/broselowtmhinkle-pediatric-emergency-system-sets](https://recalls-rappels.canada.ca/en/alert-recall/broselowtmhinkle-pediatric-emergency-system-sets)