# Major Recall Alert: Brain Premier tDCS, Brain Ultimate VNS Recalled in Canada
A significant product recall has been issued for the **Brain Premier tDCS** and **Brain Ultimate VNS** devices imported by U.S.-based Caputron. These Class II devices, manufactured in China, were inadvertently sold and shipped to Canadian customers via Caputron's e-commerce website. Health Canada flagged the recall as the importer and manufacturer lack the required **Medical Device Establishment License (MDEL)** and **Medical Device License (MDL)** to legally distribute and sell these devices in Canada. Although no functional defects have been identified, consumers are urged to act swiftly to address this licensing concern.
The recall officially began on **August 21, 2025**. Here’s what you need to know.
---
## Why This Recall is Important
Health Canada enforces strict licensing standards to ensure all medical devices meet safety, quality, and efficacy criteria before they are sold. The **Brain Premier tDCS** and **Brain Ultimate VNS** devices are Class II medical devices intended for therapeutic use. However, without proper licensing, there’s no regulatory oversight to confirm these devices meet Canadian safety and performance standards.
While no functional defects have been reported, unlicensed devices could pose potential risks due to the absence of rigorous evaluation and certification processes mandated by Health Canada. To protect public health, Health Canada has ordered an immediate recall of these products from Canadian consumers.
---
### Details of the Recall
Here are the essential facts about this recall:
- **Category**: Medical Devices (Class II)
- **Products Recalled**:
- Brain Premier tDCS
- Brain Ultimate VNS
- **Manufacturer**: Made in China
- **Importer**: U.S.-based Caputron
- **Reason for Recall**:
- Devices were sold and shipped to Canada without proper licensing (MDEL and MDL).
- Products do not meet Health Canada’s regulatory requirements.
- **Date Recall Began**: August 21, 2025
- **Affected Regions**: Canada
- **Functional Defects**: None identified at this time
- **Image of Affected Devices**:
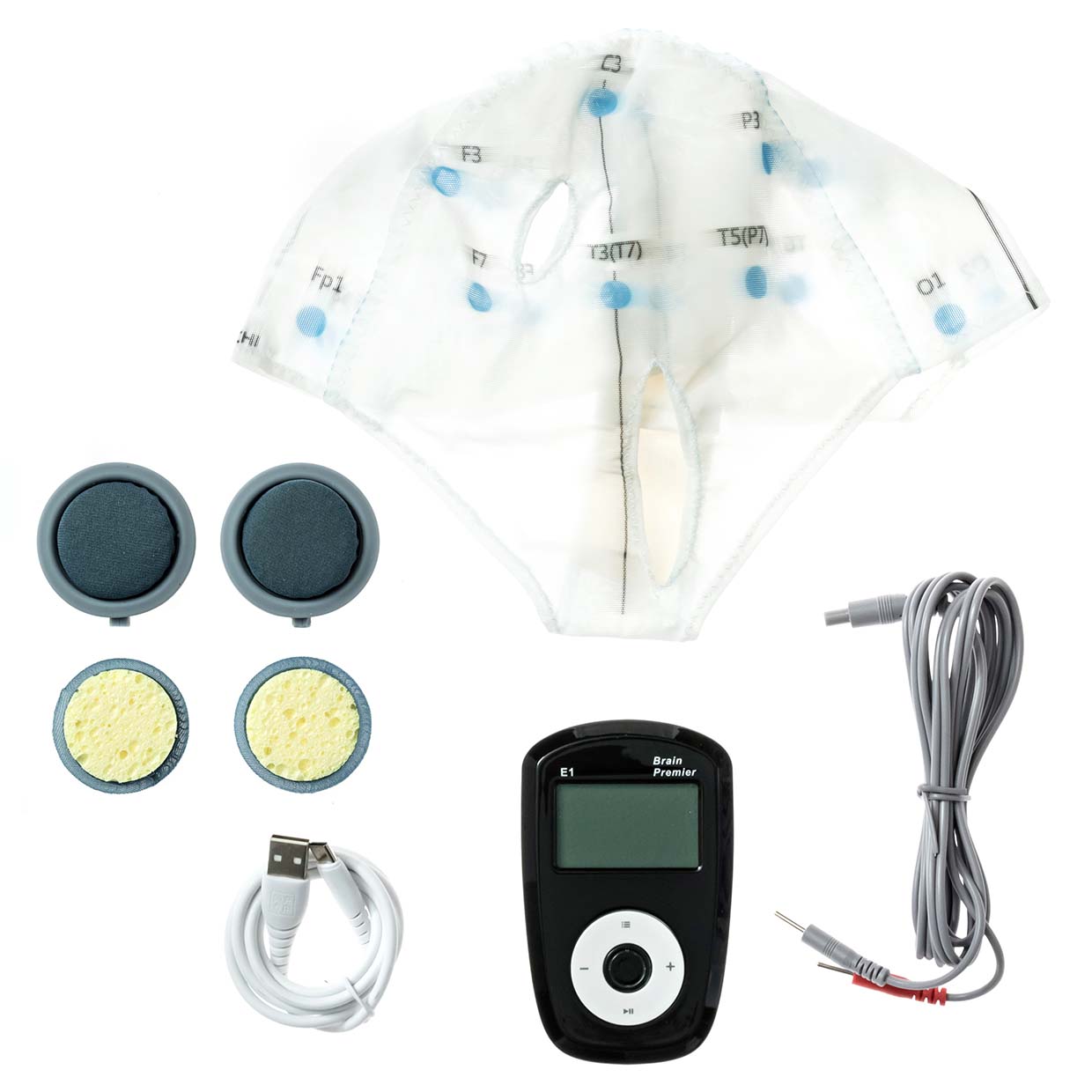
For the full Health Canada recall notice, you can visit the official source [here](https://recalls-rappels.canada.ca/en/alert-recall/caputron-brain-premier-tdcs-and-brain-ultimate-vns).
---
### What You Should Do
If you purchased either of the recalled devices, follow these steps to ensure your safety:
1. **Stop Using the Product**: Discontinue usage immediately to mitigate any potential risks.
2. **Contact Caputron**: Reach out to the importer for information on how to return the product. They may provide refunds or further instructions.
3. **Monitor Health Canada Updates**: Stay informed about new developments, especially if further safety concerns arise.
4. **Dispose of Products Safely**: If authorized, return the device or dispose of it following local electronic waste protocols.
5. **Speak With a Professional**: Consult your healthcare provider if you’ve experienced any adverse effects or have concerns about your health.
---
### Stay Safe – Get Instant Recall Alerts
Keeping up with recalls is vital for your health and safety. Don’t miss crucial updates about medical devices, food, or products that you and your family rely on. Download the **My Recalls App** today for real-time alerts directly to your phone. Ensure you’re always informed and protected – it’s free and easy to use!
[**Download the My Recalls App Now**](#)
---
The recall of the **Brain Premier tDCS** and **Brain Ultimate VNS** highlights the importance of regulatory compliance for medical devices sold in Canada. While no functional defects have been identified, consumers are encouraged to act swiftly to remove these unlicensed devices from use. For additional details on the recall, visit the official Health Canada link provided above. Stay vigilant and prioritize safety every step of the way!