# Major Recall Alert: BALLARD™ Closed Suction Systems Recalled Due to Non-Sterilized Catheter Products
**Recall Start Date: March 19, 2025**
Healthcare professionals and consumers, please take note. The BALLARD™ Closed Suction Systems, a critical tool used in medical settings, have been subject to a recall due to a potentially dangerous oversight. Certain products intended to maintain sterility through gamma irradiation were shipped to customers without proper sterilization. This poses significant health risks and warrants immediate action.
---
## Why This Recall is Important
Ensuring sterility in medical devices is vital to prevent infection and safeguard patient health. The BALLARD™ Closed Suction Catheter Systems are used to remove secretions from patients' airways in environments like hospitals and long-term care facilities. These products are designed to be sterile, as any contamination can lead to serious infections.
Unfortunately, a portion of these devices shipped to customers failed to undergo the required gamma irradiation sterilization process. This means they may no longer be deemed safe for use, putting patients’ health at risk.
---
### Details of the Recall
Here’s what we know so far about the recall:
- **Brand Name**: BALLARD™
- **Product Name**: BALLARD™ Closed Suction Systems
- **Category**: Medical Equipment – Closed Suction Catheter Systems
- **Reason for Recall**: Products intended to be sterile through gamma irradiation were shipped without undergoing the sterilization process.
- **Risk**: Use of non-sterile devices could result in increased risk of infection.
- **Recall Start Date**: March 19, 2025
- **Safety Measure**: Immediate discontinuation of product use and contact with the manufacturer.
- **Image of the Product**:
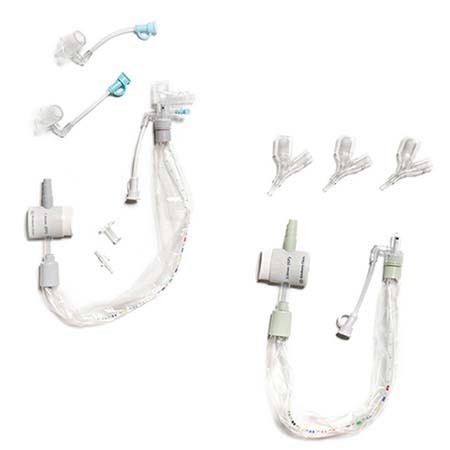
For further details, visit the official recall notice [here](https://recalls-rappels.canada.ca/en/alert-recall/ballardtm-closed-suction-systems).
---
### What You Should Do
If you are in possession of any BALLARD™ Closed Suction Systems, here's how you should respond:
1. **Check Your Product**: Verify if your product is part of the recall.
2. **Stop Using the Product**: Cease using these systems immediately to avoid any potential harm.
3. **Contact the Manufacturer**: Reach out to the distributor or manufacturer for further instructions on returning or replacing the affected product.
4. **Notify Relevant Staff**: If you’re a healthcare facility, inform all staff who may use these systems about the recall.
5. **Report Safety Concerns**: Notify Health Canada of any adverse events encountered due to this product by submitting a report.
Remember, safeguarding patient health should be the priority.
---
### Stay Safe – Get Instant Recall Alerts
Staying informed of product recalls is essential, especially for those in the healthcare industry. Ensure you act quickly when recalls are announced to protect your patients, clients, and staff from harm.
**Want to stay updated on critical recalls like this one?** Download the [MyRecalls App](https://myrecalls.app) today and get instant alerts delivered directly to your device. Don’t wait—be proactive in safeguarding safety!
Stay informed. Stay safe. Respond responsibly.
---
By staying up-to-date on product recalls, we can collectively help prevent risks and maintain the highest standards of care. Take action today to ensure you’re fully informed and equipped to handle recalls as they occur.