# Major Recall Alert: ARTEMETRIN DS (Artemether 80mg + Lumefantrine 480mg), CIPROFIT 500 (Ciprofloxacin Tablet USP 500mg) Recalled Due to Substandard and Falsified Medicines with Incorrect Active Pharmaceutical Ingredient (API) Content
The National Agency for Food and Drug Administration and Control (NAFDAC) has issued an urgent public alert regarding the recall of two widely used medications: **ARTEMETRIN DS** (Artemether 80mg + Lumefantrine 480mg) and **CIPROFIT 500** (Ciprofloxacin Tablet USP 500mg). These products, manufactured by **A.C. DRUGS Ltd** and **Impact Pharmaceutical Ltd**, have been flagged as substandard and falsified due to incorrect active pharmaceutical ingredient (API) content. Immediate action is required to safeguard public health.
## Why This Recall is Important
The integrity of medications plays a crucial role in ensuring effective treatment and protecting lives. Substandard or falsified medicines can lead to treatment failures, adverse reactions, and, in severe cases, fatalities.
The recall highlights serious pharmaceutical concerns:
- **Compromised Efficacy**: Incorrect API content in drugs like **ARTEMETRIN DS**, used for treating malaria, and **CIPROFIT 500**, an antibiotic, can result in failed treatments and worsen patient conditions.
- **Potential Harm**: Substandard medicines may expose consumers to unknown substances, exacerbating health risks.
- **Public Safety Threat**: The sale of counterfeit medications undermines trust in healthcare systems and poses a significant global health issue.
Given the severity of the risks, NAFDAC urges immediate action to prevent further harm.
## Details of the Recall
Here’s everything you need to know about this critical recall:
- **Affected Products**:
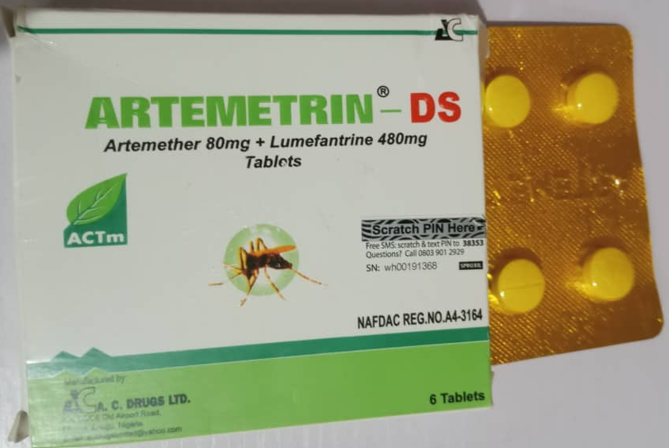
- **ARTEMETRIN DS (Artemether 80mg + Lumefantrine 480mg)**
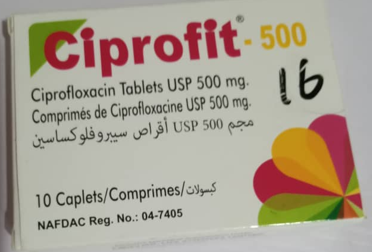
- **CIPROFIT 500 (Ciprofloxacin Tablet USP 500mg)**
- **Manufacturers**:
- **A.C. DRUGS Ltd**
- **Impact Pharmaceutical Ltd**
- **Reason for Recall**:
- Substandard and falsified medicines containing incorrect API content.
- **Date Announced**: **September 29, 2023**
- **Images**: Check product images on the official NAFDAC website:
- 
- 
For more details, visit NAFDAC’s official alert: [NAFDAC Recall Notification](https://nafdac.gov.ng/public-alert-no-030-2025-sale-of-confirmed-substandard-and-falsified-artemetrin-ds-and-ciprofit-500/).
## What You Should Do
NAFDAC has issued clear instructions for individuals, healthcare providers, and retailers to mitigate risks:
- **Stop Usage Immediately**: If you have ARTEMETRIN DS or CIPROFIT 500 in your possession, do not consume them.
- **Return the Products**: Submit any stocks of the recalled medications to the nearest **NAFDAC office** as soon as possible.
- **Seek Medical Help**: If you’ve experienced adverse effects from these medicines, consult a healthcare professional without delay.
- **Report Suspicious Medicines**: Use NAFDAC’s reporting platforms to notify them about any substandard or falsified drugs.
Your prompt action can save lives. Share this information with your family and community to enhance awareness and prevent further exposure to these hazardous medications.
## Stay Safe – Get Instant Recall Alerts
In today’s fast-paced world, staying informed about product safety is vital. Protect yourself and your loved ones by downloading the **NAFDAC app** for instant updates on recalls, drug safety alerts, and more.
- **Download now to access:**
- Real-time recall notifications.
- Verified product authenticity checks.
- Easy reporting of suspicious medicines.
Visit [NAFDAC’s official website](https://nafdac.gov.ng/) to learn more and stay vigilant against substandard pharmaceuticals.
Your health is your priority. By staying updated and informed, you play an essential role in promoting safer, healthier communities.