# Major Recall Alert: Ambu® SPUR®II Adult and Pediatric Resuscitator Recalled Due to Blocked Manometer Port
The Ambu® SPUR®II Adult Resuscitator and Ambu® SPUR®II Pediatric Resuscitator have been officially recalled due to a critical defect affecting the devices' manometer port. This defect has rendered the attached manometer non-functional, causing increased risks related to oxygen delivery and patient safety.
Although no patient-related incidents have been reported, healthcare providers using these devices should take immediate action to ensure patient safety and optimal care. Below, we provide complete details about the issue, its risks, and what steps you need to take.
---
## Why This Recall is Important
Medical equipment must function flawlessly, especially in critical care settings. For the defective Ambu® SPUR®II devices:
- **Reported Issue**: A blocked manometer port makes the attached manometer non-functional.
- **Safety Risks**:
- **Barotrauma**: If the pressure-limiting valve is overridden, users may unintentionally expose patients to excessive airway pressure.
- **Temporary Hypoxia Risk**: Delays in procedures caused by uncertainty with the device could lead to temporary oxygen deprivation (hypoxia).
- **Affected Use**: The issue compromises pressure monitoring via the manometer port, leaving users unable to gauge and control pressure levels precisely.
Despite the defect, the device's core oxygen delivery capabilities remain functional. Still, improper pressure management increases significant risks in life-saving situations.
---
## Details of the Recall
- **Products Involved**:
- **Ambu® SPUR®II Adult Resuscitator**
- **Ambu® SPUR®II Pediatric Resuscitator**
- **Problem Identified**: A blocked manometer port prevents the attached manometer from functioning. This manufacturing defect has been reported by users five times, although no patient incidents were cited.
- **Risks from Defective Devices**:
- Inability to track internal airway pressure.
- Potential for improper pressure control if the pressure-limiting valve fails or is overridden.
- Delayed procedures stemming from uncertainty when using a device missing critical functionality.
**Recall Start Date**: July 14, 2025
- **Images of the Product**:
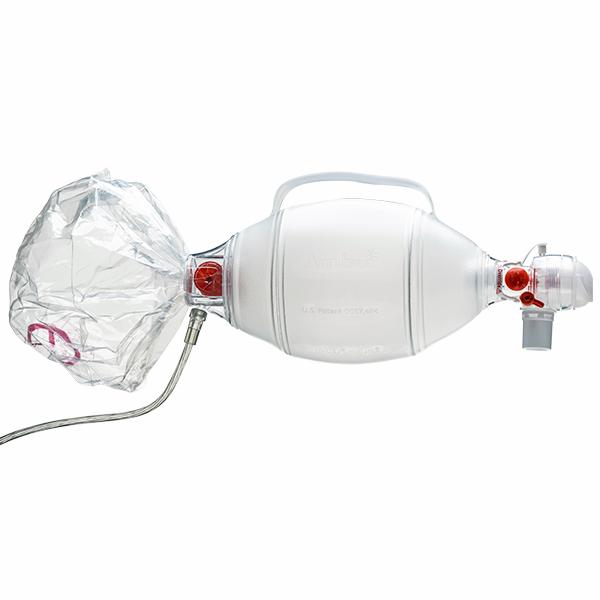
For official information about this recall, visit [the Canadian Recall Alert page here](https://recalls-rappels.canada.ca/en/alert-recall/ambur-spurrii-adult-and-pediatric-resuscitator).
---
## What You Should Do
If you use or distribute the Ambu® SPUR®II Adult or Pediatric Resuscitator, follow these steps immediately:
- **Review Inventory**: Identify if your facility holds the affected devices.
- **Cease Use**: Stop using the defective Ambu® SPUR®II devices to prevent risks to patients.
- **Contact the Manufacturer**: Reach out to Ambu® for guidance on replacement or repair.
- **Report Safety Concerns**: Notify Health Canada or the manufacturer directly if you identify additional product issues.
Keeping patients safe is of utmost importance. Always use functional and well-maintained medical devices in critical care environments.
---
## Stay Safe – Get Instant Recall Alerts
Product recalls like this one demonstrate why it’s essential to stay updated on medical equipment safety. Delayed awareness can compromise both patient and operator safety. To stay informed:
- **Sign Up for Alerts**: Get real-time recall notifications directly on your phone or email.
- **Download Our App**: Access instant recall news with ease and ensure your team is always prepared.
[**Click here to download the app now!**](#)
Stay proactive, informed, and prepared to ensure the safest care standards in your practice. Always monitor product recalls for any updates and ensure compliance with issued safety protocols.
For further details, visit [the official recall announcement](https://recalls-rappels.canada.ca/en/alert-recall/ambur-spurrii-adult-and-pediatric-resuscitator).